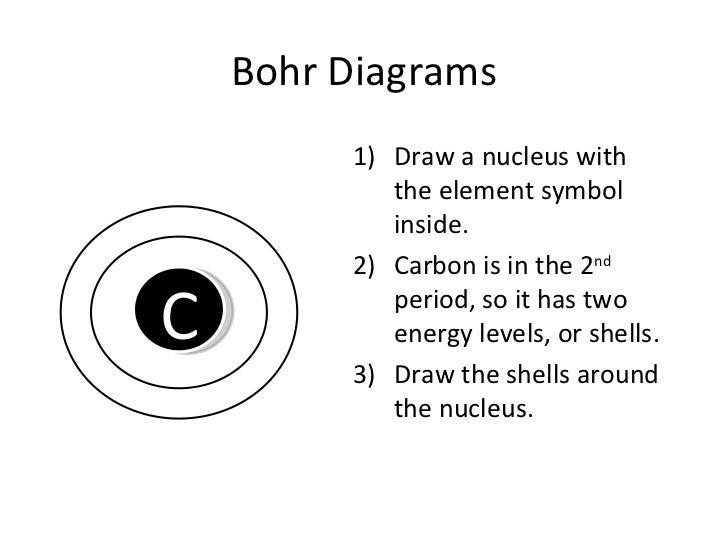
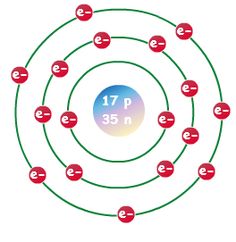
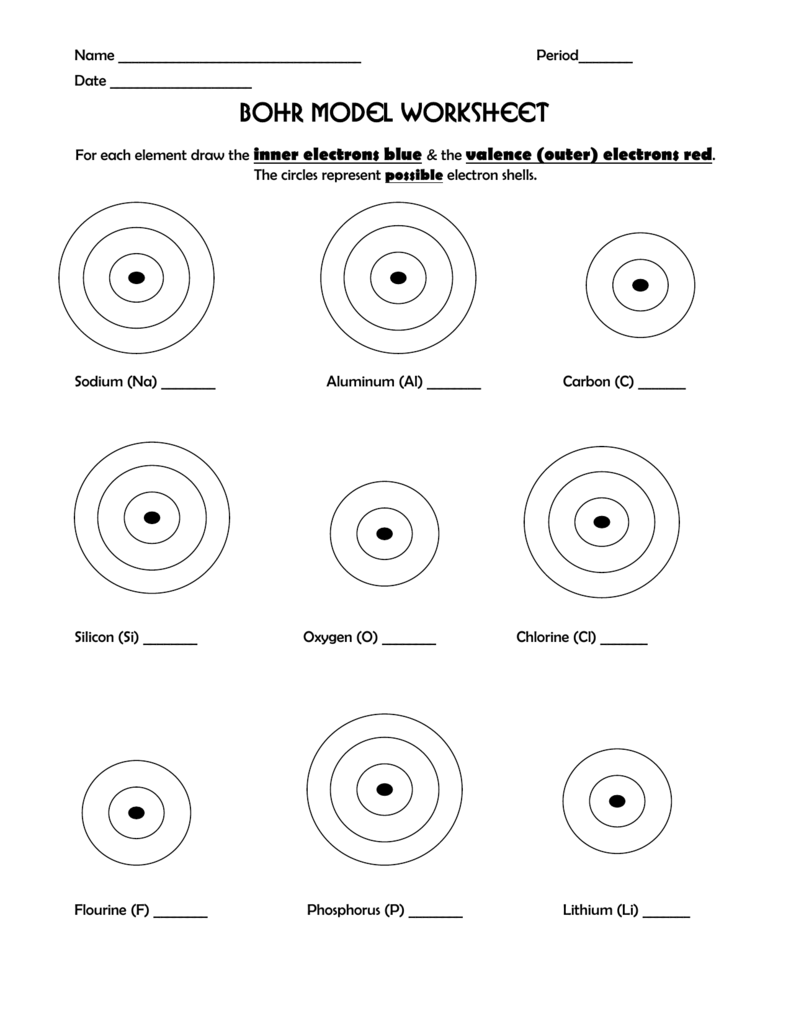
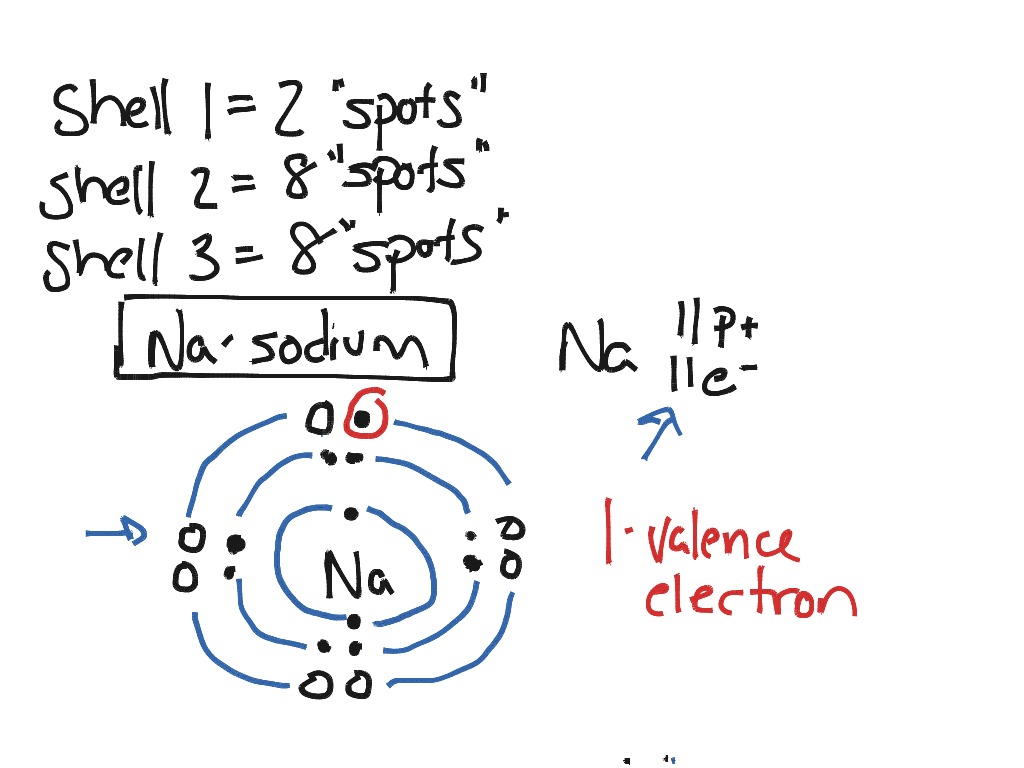
Newton sc 10 how to draw a bohr model atom and ion by diane newton april 3 2013. Draw the electrons in a bohr model electrons are housed in shells and these shells are drawn as circles with the nucleus in their center.
Each shell can house up to a fixed number of electrons.

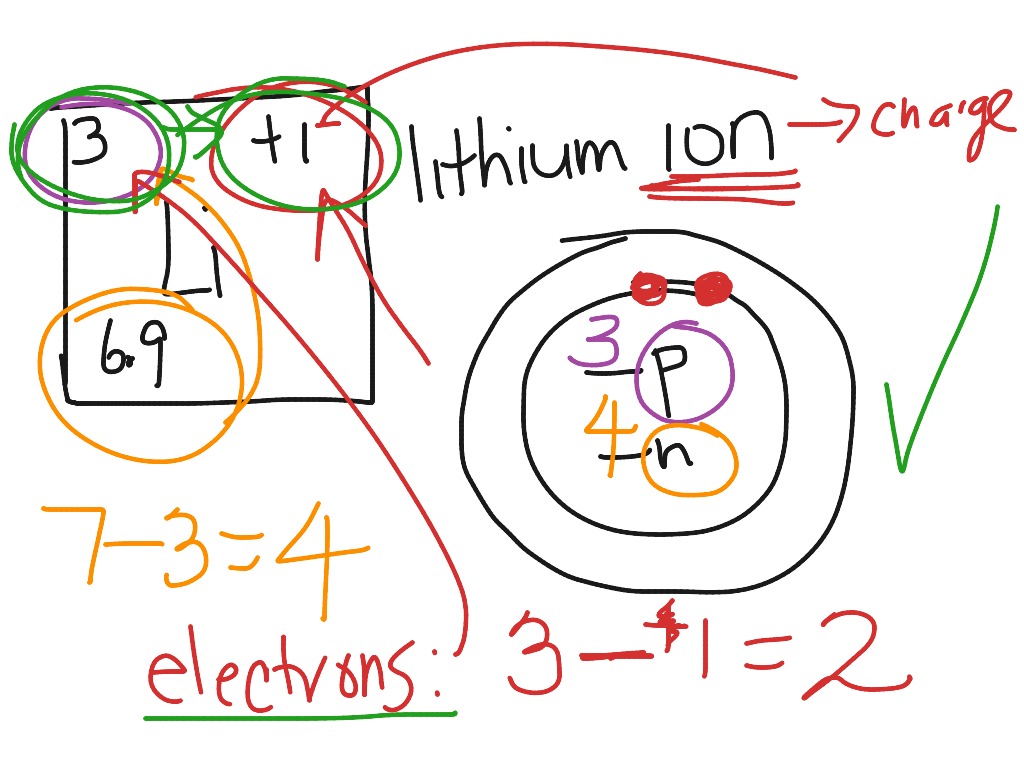
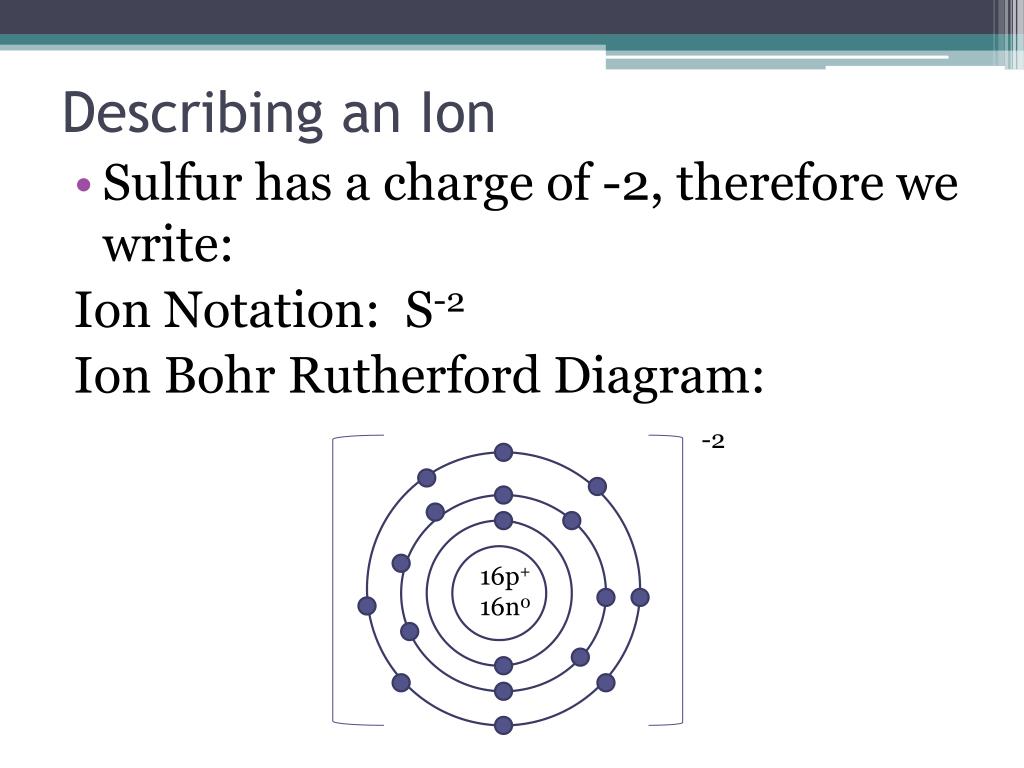
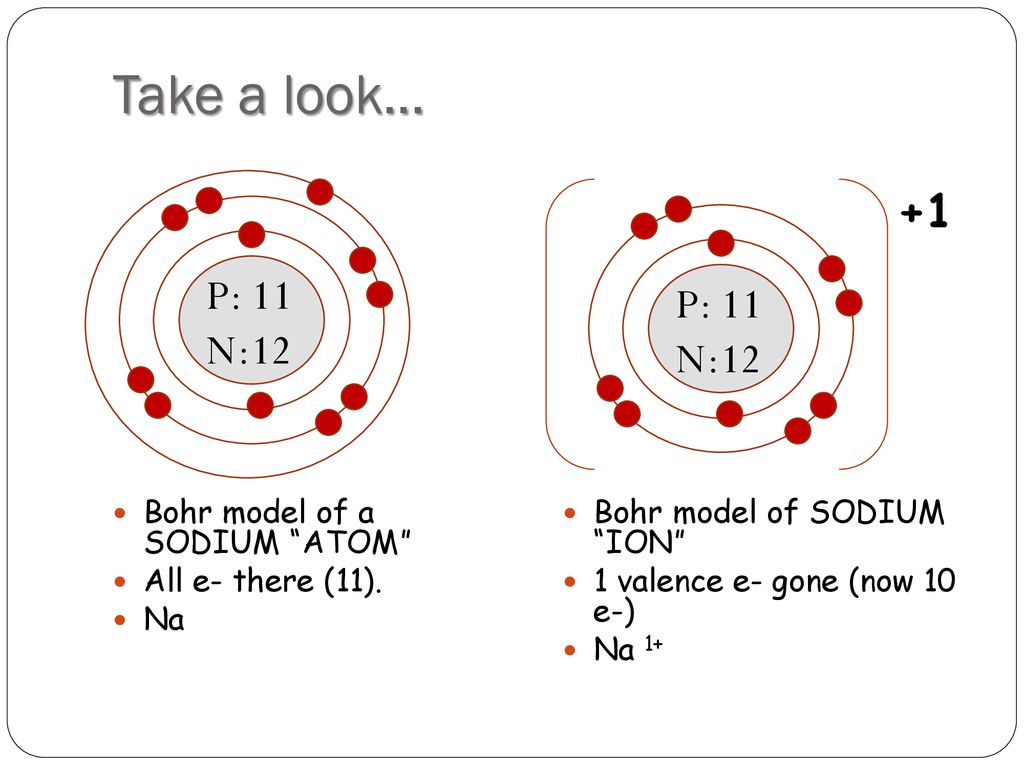
How to draw a bohr model for an ion.
When you draw the bohr diagram of the ion you will find that the ion satisfies the octet rule.
The first shell can hold two electrons the second one can hold eight electrons the third one can hold 18 and so on.
These orbits form electron shells or energy levels which are a way of visualizing the number of electrons in the various shells.
Aluminum bohr model best of sulfide lewis dot structure fresh electron newton sc 10 how to draw a bohr model atom and ion science chemistry showme bohr diagrams 1 draw bohr model practice with cations anions and isotopes.
From the diagram below you can see that ion will be a tetrahedral.
Po4 3 is the phosphate ion if you add the total number of valence electrons you will get 32 as an answer.
The bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific distances from the nucleus figure pageindex1.
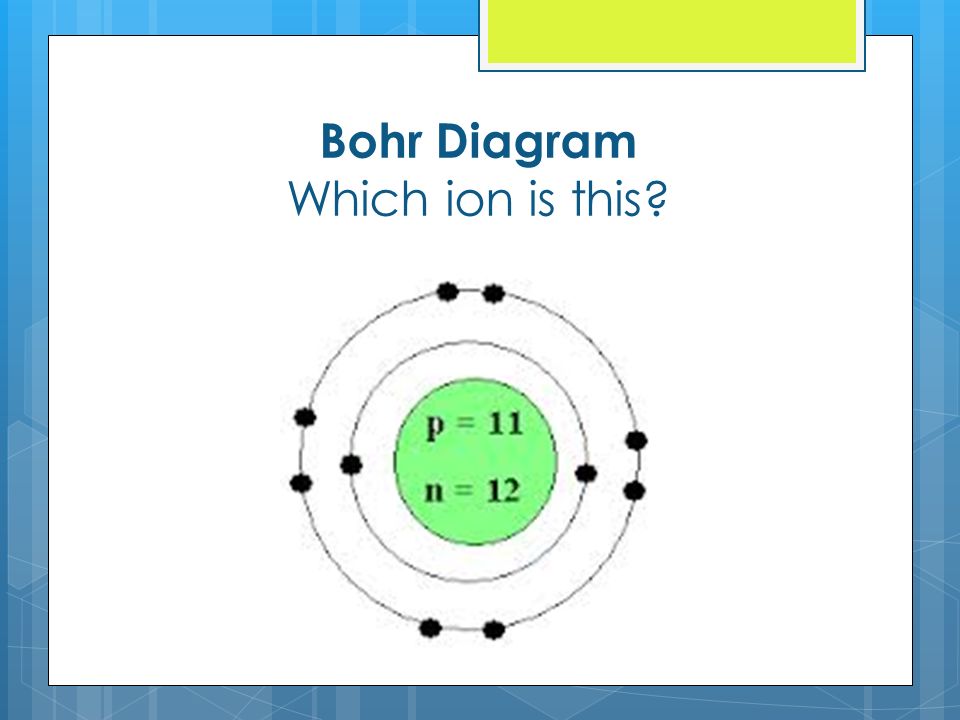
Atom vs ions comparing bohr diagrams chemistry lesson youtube.








No comments:
Post a Comment